Key Takeaway
Six months of transdermal nicotine improved attention and memory in non-smoking adults with mild cognitive impairment, with no significant safety concerns.
Summary
This pilot clinical trial tested transdermal nicotine patches (15mg/day) in 67 non-smoking adults with mild cognitive impairment over 6 months. The nicotine group showed significant improvements in attention, memory, and psychomotor speed compared to placebo.
Notably, participants were non-smokers, demonstrating cognitive benefits independent of addiction. The treatment was well-tolerated with no significant adverse effects or dependency development at study end.
This is one of the few studies examining longer-term nicotine use in non-smokers, supporting its potential as a cognitive enhancer with an acceptable safety profile.
Methods
- Double-blind, placebo-controlled RCT
- 67 non-smoking adults with MCI
- 6-month treatment duration
- Transdermal nicotine 15mg/day
Key Results
- Improved attention measures
- Enhanced memory performance
- Better psychomotor speed
- No significant adverse effects
- No dependency at study end
Figures
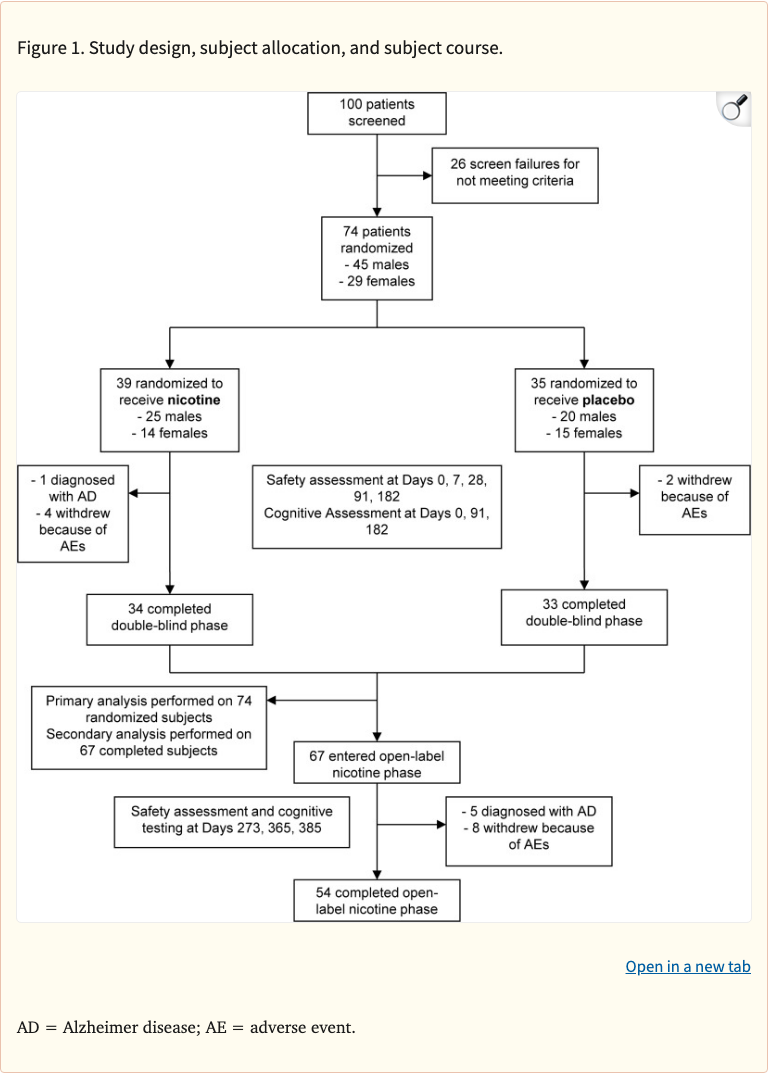 Figure 1
Figure 1
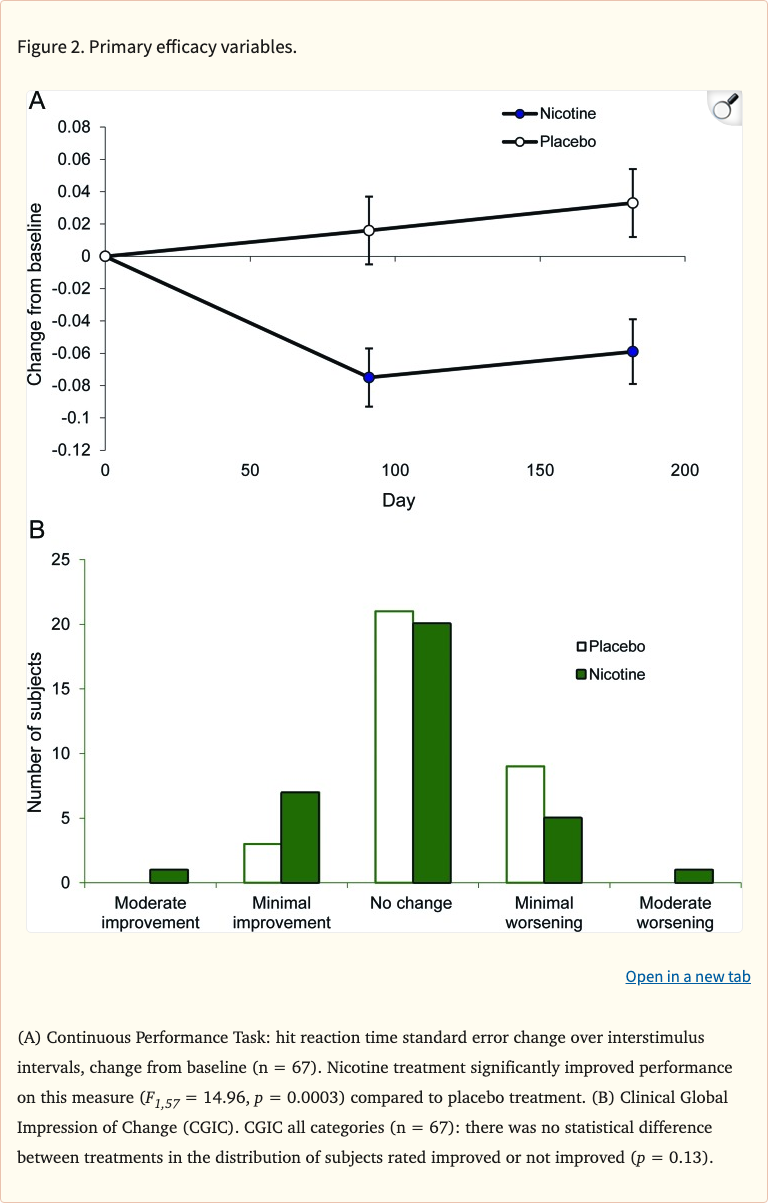 Figure 2
Figure 2
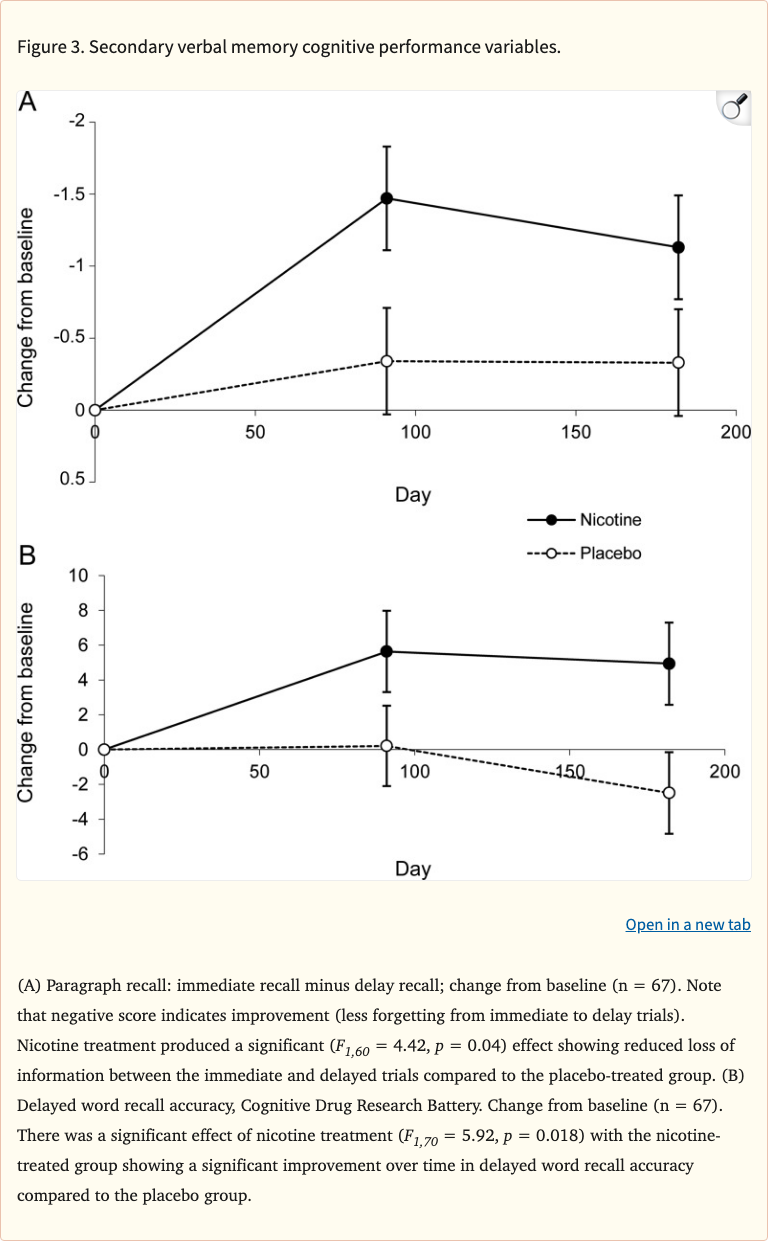 Figure 3
Figure 3
Limitations
- Pilot study, relatively small sample
- MCI population may differ from healthy adults
- Single dose level tested
- Long-term effects beyond 6 months unknown
